Heat, Internal Energy and Work
Heat, Internal Energy and Work: Overview
This Topic covers sub-topics such as Internal Energy, Internal Energy in Thermodynamics, Work Done by Thermodynamic System and, Distinction between Heat and Internal Energy
Important Questions on Heat, Internal Energy and Work
When work done by a system was , the increase in the internal energy of the system was . The heat 'q' supplied to the system was
Consider the following statements-
a) is a state function in isochoric process.
b) is state function in isobaric process.
c) Work done by the system will be zero in adiabatic free expansion.
Correct among the following is/are
What is the distinction between heat and internal energy?
At a given temperature, the specific heat of a gas at constant pressure is always greater than its specific heat at constant volume.
An ideal is filled in a closed, rigid and thermally-insulated container. A coil of resistance and carrying a current of supplies heaT to the gas. The change in the internal energy of the gas after minutes will be:
For an ideal gas, its internal energy is the function of its:
Write the difference between.heat and internal energy
Heat and internal energy are path function
Define internal energy and heat?
The heat supplied to a system is used up entirely by the system in doing work on the environment during
Heat transferred to the surrounding will increase the internal energy of a system.
The standard internal energy change of the cell reaction will be
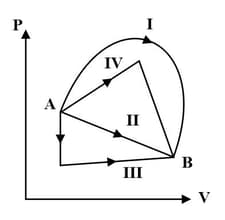
A thermodynamical system undergoes cyclic process as shown in figure. Work done by the system is _____ (in letters).
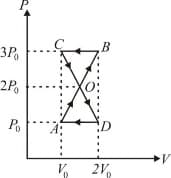
An ideal gas undergoing a change of state from to through four different paths, and as shown in the diagram that lead to the same change of state, then the change in internal energy is same in all the cases.
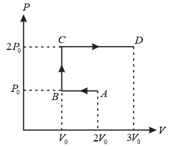
Calculate the work done by an ideal gas in the thermodynamic process depicted in the adjoining graph.
Which is contained in a body in a greater amount: heat or internal energy?
Heat transferred to the surrounding will increase the internal energy of a system.
What was the primary form of rent that peasants had to pay to their lords?
What is the primary agent of mechanical weathering?
What is the primary motive behind international business?